5901 Botham Jean Blvd, Dallas, TX 75215
Why Is It Important to Know How to Decommission an MRI Machine?
February 5, 2026A single mistake during MRI decommissioning can lead to EPA fines exceeding $70,000 per day. These sophisticated medical imaging systems present extraordinary challenges beyond typical equipment retirement. The stakes are high for healthcare facilities and recycling providers handling these complex machines in 2026, as environmental oversight reaches new heights in the medical sector.
MRI machines contain hazardous materials, including mercury and beryllium, that require specialized handling protocols. The powerful superconducting magnets operate at magnetic field strengths thousands of times greater than Earth’s magnetic field. Patient data stored within computer systems must meet strict HIPAA compliance standards during disposal. Each component demands precise technical expertise to prevent environmental contamination and regulatory violations.
What initial planning is required for MRI removal?
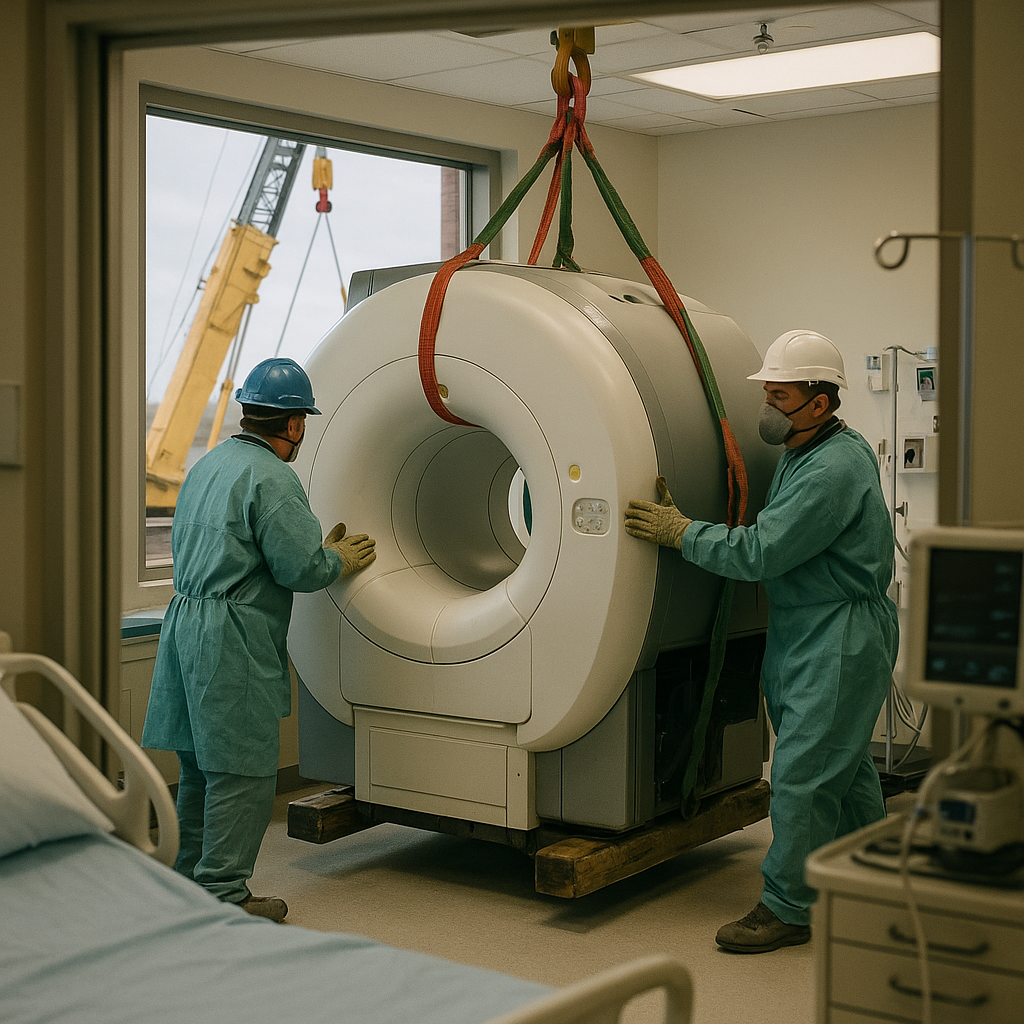
Proper MRI removal begins with comprehensive planning long before any equipment is moved. Detailed site assessments help evaluate every aspect of the removal process. This planning phase determines whether the project runs smoothly or faces costly delays. In 2026, many Texas facilities are adopting “smart decommissioning” plans that integrate logistics with real-time asset recovery tracking.
Route assessment is crucial for successful MRI removal. All doors and windows along potential removal paths are measured to ensure adequate clearance. Professional removal teams thoroughly examine each possible route, calculating the exact dimensions needed for safe equipment transport. This often involves structural engineering reviews to ensure floor loading capacities can handle the concentrated weight of the magnet during transit.
Facility manager coordination
Close coordination with facility managers addresses operational concerns during removal. These discussions cover potential service interruptions and scheduling constraints that could affect the healthcare facility’s daily operations. Facility managers offer valuable insights about building limitations and patient flow patterns, ensuring that the removal of heavy imaging equipment does not compromise critical care services.
The consultation process determines whether structural modifications are needed. For larger MRI systems, doors or windows may need to be removed to accommodate equipment dimensions. Some facilities have knockout panels specifically designed for equipment removal, which are evaluated during initial planning to minimize reconstruction costs after the project is complete.
Infrastructure protection measures
Protecting the facility during MRI removal requires careful preparation. Protective materials are laid down to safeguard floors, walls, and other surfaces from potential damage. This includes specialized floor coverings and wall protection systems designed for heavy equipment transport. Crews assess ceiling heights and structural elements that could be affected, identifying vulnerable areas like doorframes and narrow passages that need extra reinforcement.
Advanced metallurgical recovery: Niobium and titanium alloys
While most recyclers focus on the bulk steel and copper found in medical equipment, 2026 industrial standards prioritize the recovery of high-value superconducting alloys. The “heart” of the MRI is its superconducting coil, which contains thousands of miles of specialized wire.
The value of niobium-titanium (NbTi)
MRI magnets rely on niobium-titanium alloys to achieve superconductivity. These materials are incredibly rare and expensive to mine. By utilizing specialized de-manufacturing techniques, industrial recyclers can isolate the NbTi filaments from the copper matrix they are embedded in. In 2026, the demand for these alloys in the aerospace and renewable energy sectors has driven their recovery value to record highs, often reaching $15 per pound when processed correctly. This recovery is a cornerstone of the medical circular economy.
Separating the superconducting matrix
Recovering these alloys requires a sophisticated chemical and mechanical process. Because the niobium-titanium is bonded to high-purity copper for thermal stability, the wire must be shredded and subjected to density separation or specialized leaching. Facilities that can perform this separation provide a significantly higher return on investment for healthcare organizations, turning a decommissioned asset into a valuable source of rare materials for the next generation of high-tech manufacturing.
Navigating 2026 environmental and safety mandates
The regulatory landscape for medical equipment disposal in Texas has evolved significantly as we enter 2026. Healthcare providers must now navigate a “cradle-to-grave” responsibility that includes detailed carbon footprint reporting and material traceability.
Compliance with the Texas Medical Waste Act of 2025
New state mandates now require healthcare facilities to provide a “Full Cycle Disposition Report” for any diagnostic equipment weighing over 2,000 pounds. This law ensures that heavy medical scrap is not exported to unregulated international markets. Professional recycling partners manage this documentation, providing certified weight tickets and destruction certificates that prove every pound of steel, lead, and copper was processed at a domestic, permitted facility. Failure to produce these reports during a state audit can result in administrative penalties exceeding $25,000 per violation.
Cryogenic safety and helium conservation
Helium is a finite global resource, and the 2026 market has seen unprecedented price volatility. Decommissioning protocols now emphasize “Helium Capture” rather than simple venting. Modern quenching procedures allow for the reclamation of liquid helium, which can then be purified and reused in other medical or industrial applications. Professional teams use specialized vacuum-insulated piping and cryo-containers to capture this gas, reducing the environmental impact and potentially providing a credit toward the decommissioning costs.
What is the step-by-step process for decommissioning?
Initial preparation and safety assessment
The decommissioning process starts with comprehensive planning by technicians who understand both technical complexities and safety requirements. These professionals conduct detailed assessments of the equipment’s current state, identifying power sources, cryogenic systems, and potential hazards. Documenting serial numbers and system configurations ensures total accountability. Safety protocols must be established before any physical work begins, including the verification of cryogenic PPE and non-ferrous toolkits to prevent accidents in the presence of any residual magnetic field.
Electrical system shutdown
The power-down sequence follows strict lockout/tagout procedures to prevent accidental energization. Technicians systematically disconnect all electrical connections, starting with primary power sources. Each circuit is properly isolated and tested to confirm a zero-energy state. Control panels and transformers are evaluated for reuse potential, as they often contain heavy copper windings that fetch premium prices at the recycling scale.
Cryogen management and field reduction
Managing cryogenic materials is a critical phase. Liquid helium systems require specialized handling due to their extremely low temperatures and potential for rapid expansion. Technicians follow established protocols for safe cryogenic handling to prevent oxygen deficiency in the magnet room. The magnet quench must be performed gradually to allow the magnetic field to dissipate safely while preserving the internal support structures for easier dismantling.
| Aspects | Details |
|---|---|
| Handling Protocols | Use designated transfer equipment, transfer slowly to minimize boiling, and avoid direct contact with uninsulated surfaces. |
| Personal Protective Equipment (PPE) | Cryogenic-resistant gloves, face shields, safety goggles, and non-absorbent protective clothing. |
| Key Regulatory Bodies | FDA, OSHA, DOT, and TCEQ (Texas Commission on Environmental Quality). |
| Compliance and Safety Standards | FDA cGMP, OSHA training requirements, and DOT Hazardous Materials Regulations. |
Component disassembly and separation
The systematic disassembly phase focuses on component separation to maximize material recovery. Technicians identify and separate different material types, including copper, aluminum, and rare earth magnets. This careful sorting ensures maximum diversion from waste streams. Hazardous materials, such as mercury switches or lead shielding, receive special attention and follow specific disposal protocols to prevent environmental contamination.
Transportation and final documentation
The main magnetic component requires specialized packaging for secure transport. Heavy-duty wrapping protects the facility during the load-out, and custom crating is used for large components. Transportation logistics ensure proper vehicle selection for the extreme weight. Final documentation—including material manifests and recycling certificates—provides a complete chain of custody that supports regulatory compliance and verifies proper materials management.
What are the key safety and compliance considerations?
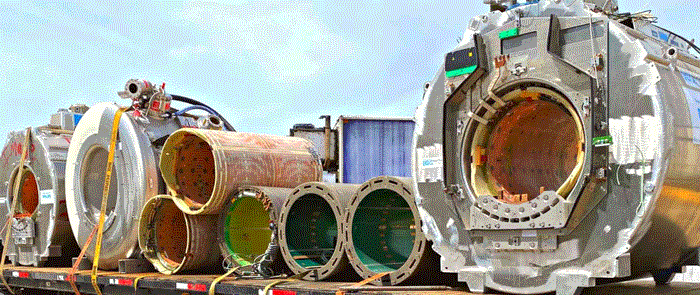
Data security and HIPAA compliance
The Health Insurance Portability and Accountability Act (HIPAA) sets strict guidelines for handling protected health information stored on MRI computer modules. Organizations must adopt technical safeguards to ensure data confidentiality. Data destruction protocols require the permanent elimination of all patient records using high-intensity degaussing or physical shredding. The HIPAA Security Rule mandates that covered entities accurately assess vulnerabilities, including establishing device management procedures for the entire lifecycle of imaging hardware.
Environmental safety and EPA regulations
Electronic storage devices and medical components contain hazardous materials that need specialized handling under EPA guidelines. Mercury, beryllium, and rare earth elements pose significant risks if not managed properly. EPA regulations require strict protocols for the handling, transportation, and disposal of hazardous medical scrap. Organizations must maintain detailed tracking documentation to ensure full accountability. Environmental violations can lead to substantial daily penalties, making professional compliance critical for business continuity in the healthcare sector.
What are the options for an MRI machine after removal?
Recycling for material recovery
Recycling is the most environmentally responsible option for decommissioned MRI machines. These devices contain substantial quantities of valuable metals, including high-purity copper and aluminum. The circular economy benefits of MRI recycling extend to the reintegration of rare earth elements into new manufacturing processes, reducing the demand for destructive mining operations.
Direct resale and consignment
Direct resale is a viable option for systems that retain functional value. Facilities must consider age and usage history when evaluating resale potential. Consignment offers a middle ground, where a professional recycler removes the system and manages the sales process while ownership remains with the facility. This approach offers timeline flexibility, allowing for new equipment installation without waiting for a buyer to be identified.
Strategic parting out and trade-ins
Parting out involves the disassembly of systems to sell individual components like gradient coils or RF systems separately. This is effective for older units where complete resale is impractical. Alternatively, trade-in programs apply the existing system’s value toward replacement purchases, reducing administrative complexity and coordinating removal and installation activities into a single project window.
Professional disposal services
When systems reach the end of their useful life with no remaining resale value, professional disposal is the appropriate choice. This involves safe dismantling, hazardous material handling, and compliant waste management. Even in disposal scenarios, scrap metal recovery can offset removal costs while supporting responsible resource management. Certified recyclers provide the necessary certificates of destruction and waste tracking records essential for environmental reporting.
Conclusion: The importance of professional MRI decommissioning
Decommissioning an MRI machine is a high-stakes task that demands specialized expertise to ensure safety, data privacy, and environmental compliance. This complex process involves managing powerful superconducting magnets, handling hazardous materials like liquid helium, and systematically disassembling the machine to maximize material recovery. From initial planning and safe shutdown to compliant recycling and disposal, each step requires careful execution by professionals knowledgeable in technical and regulatory challenges.
Partnering with a professional decommissioning service is the most effective way to manage these complexities and foster a more sustainable healthcare industry. Professional services ensure regulatory compliance while recovering valuable materials like rare earth magnets and copper components, transforming disposal costs into potential revenue.
For healthcare facilities seeking responsible and compliant MRI decommissioning practices, contact Okon Recycling at 214-717-4083.
